Sustainability
New technologies

Basic chemicals such as hydrogen and methanol are responsible for around 70 percent of greenhouse gas emissions of the european chemical industry – but they are also the indispensable starting point for all the innovative products that enable our customers to protect the climate and that make our everyday lives easier. That is why our research focuses on basic chemicals.

The most important climate-friendly technologies on which BASF is working at full speed include the electrically heated steam cracker furnace for the production of basic chemicals and processes for the production of hydrogen such as methane pyrolysis and water electrolysis. Clean hydrogen is a key to the success of the transformation toward climate-friendly chemistry, mobility and heating. Researchers have also developed a process to produce methanol without any greenhouse gas emissions and we want to investigate the use of storage processes for CO2.
Our most important new technologies

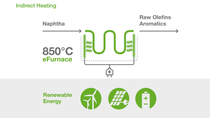
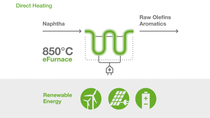
eFurnace Technology:
World's first large-scale electrically heated steam cracking furnace
Together with SABIC and Linde, BASF started up the world's first demonstration plant for large-scale electrically heated steam cracking furnaces. The demonstration plant with 6 megawatts input of renewable electrical energy tests material behavior and process on an industrial scale and is fully integrated into the existing steam crackers in Ludwigshafen.
Steam crackers play a central role in the production of basic chemicals and require a significant amount of energy to break down hydrocarbons into olefins and aromatics. Typically, the reaction is conducted in furnaces at temperatures of about 850 degrees Celsius. Up to now, these temperatures have been reached by using conventional fuels. The demonstration plant aims to show that continuous olefin production is possible using electricity as a heat source.
By using electricity from renewable sources, the new technology has the potential to reduce CO2 emissions of one of the most energy-intensive production processes in the chemical industry by at least 90% compared to technologies commonly used today.

In two separate demonstration furnaces, two different heating concepts are tested:
To support the development of the novel furnace technology, the project was granted funding by the German Federal Ministry for Economic Affairs and Climate Action under its “Decarbonization in Industry” funding program.


Further Information
Climate neutrality with clean hydrogen
For BASF, the use of clean hydrogen is a key element in reducing greenhouse gas emissions. In Europe, for example, BASF is one of the largest hydrogen producers. At our main plant in Ludwigshafen alone, we produce around 250,000 metric tons of hydrogen per year. The gas is a key and irreplaceable raw material for important products such as ammonia and is found in many consumer products from chewing gum to plastics. Hydrogen is mostly produced from hydrocarbons such as natural gas by steam reforming, which involves high CO2 emissions (about 9 to 10 tons of CO2 per ton of hydrogen). This makes hydrogen production one of the largest CO2 emitters in the chemical industry.
In order to produce hydrogen without CO2 in the future, BASF is relying in parallel on two processes: commercially available water electrolysis and methane pyrolysis, for which BASF is developing a new process technology. In order to further expand CO2-free hydrogen production, sufficient electricity based on renewable energy must be available
While hydrogen is used as a material in the chemical industry, it can be used as an energy carrier (mobility, building heating) in other areas of application. Since low-emission hydrogen is in short supply, it needs to be prioritized for those areas in which its use is essential.

Clean hydrogen: Methane pyrolysis
The chemical industry needs large quantities of hydrogen. For instance, BASF uses it as a reactant for ammonia synthesis. Because hydrogen is indispensable for carrying and storing energy in numerous sustainable future applications, it will continue to grow in importance.
Steam reforming is currently the most important industrial-scale procedure for producing hydrogen from natural gas or coal. However, this process releases considerable quantities of CO2.
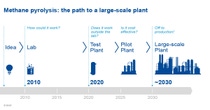
As part of its Carbon Management program, BASF is working together with cooperation partners in a project funded by the Federal Ministry of Education and Research (BMBF) to develop methane pyrolysis, a methane pyrolysis technology for producing climate-friendly hydrogen from natural gas. Here, methane or natural gas, which mainly consists of methane, is split directly into its components of hydrogen and solid carbon. The process uses comparatively little energy and, if it is run using electricity from renewable resources, is even CO2-free. Compared to other processes for emission-free hydrogen production, methane pyrolysis requires only around one-fifth as much electrical energy. We have been operating a test plant for methane pyrolysis at the Ludwigshafen site since 2021. Today, the optimal functionality of the plant and heating concept has been confirmed by successful continuous operation of the reactor. The next step is to expand the test plant.
We are working at high pressure on methane pyrolysis, because feasible solutions for clean hydrogen are needed. It is a particular challenge for us to help shape this path to a low carbon chemistry.“
It is still unclear how the granular carbon from our pyrolysis will be used. In general, there are numerous markets for solid carbon, especially for high purity carbon, e.g. aliminium or steel industries.
Storage is also conceivable in principle, because our pyrolysis carbon is not a hazardous substance and can be stored in a stable manner. The various approaches are being investigated in the current project.

CO2-free hydrogen production: Water electrolysis

Climate-friendly hydrogen plays an important role in the transformation of the chemical industry toward climate neutrality. BASF is open to technology and is focusing on various hydrogen technologies that will produce climate-friendly hydrogen from electricity from renewable sources in the future. In addition to methane pyrolysis, this includes water electrolysis.
In water electrolysis, water is split into hydrogen and oxygen using electricity. Since the energy here is introduced by electricity and not by an oxidation process, i.e. combustion, electricity consumption is high.
In cooperation with Siemens Energy, we are building a water electrolysis plant at the Ludwigshafen site with a capacity of 54 megawatts and integrate it into the Verbund system. The project Hy4Chem-EI is funded by the Federal Ministry for Economic Affairs and Climate Action and the State of Rhineland-Palatinate. The climate-friendly hydrogen will be used as a raw material to manufacture products with a low CO2 footprint. It will also be used to a smaller extend for regional mobility concepts in the metropolitan Region Rhine-Neckar.

Carbon Capture, Storage and Utilization
Our goal is to prevent CO2 emissions from occurring in the first place. However, even with a full transition to renewable power and adoption of new production technologies, it will be next to impossible to completely avoid CO2 emissions in some areas. These residual emissions, often referred to as "hard-to-abate", which can demonstrably only be avoided with considerable technical effort or at extremely high cost, significantly threaten the competitiveness of our production or the existence of certain products. In addition, bringing some of the new, low carbon emitting technologies to a commercial scale will take time. That’s why we need Carbon Capture and Storage (CCS) as a transition technology that, depending on the availability of infrastructure, could help to achieve our climate targets while remaining competitive. The key word here is "transitional". We cannot rely on CCS as a permanent solution and continue to operate in a "fossil" world. To reach net zero and reduce the atmospheric CO2 concentration, we have to move towards using CO2 as a raw material (CCU).
BASF is currently looking at various CCS and CCU projects for our sites in Europe, the U.S. and Asia. One example is Project Kairos@C in Antwerp. The goal is to significantly reduce CO2 emissions at the industrial cluster in the port of Antwerp. The project is co-funded by the European Union and the Flemish Government.

Verbund site Antwerp: CCS is a mature drop-in
solution for large-scale process emission abatement.


CO2-free production of Methanol
Methanol is an important feedstock for the chemical industry. BASF researchers therefore worked on a new climate-friendly process for producing this basic chemical with the aim of not only reducing CO2 emissions, but also not emitting any CO2 throughout the entire process. Process development as part of the Carbon Management R&D program has been completed and BASF is currently reviewing all options for use.
The process
In BASF's new process, syngas is produced by partial oxidation of natural gas or biogas, which produces no CO2 emissions. While the process steps of methanol synthesis and distillation could be adopted almost unchanged, inventiveness was required when it came to combining and processing the waste gas streams generated here. They are first burned with pure oxygen (oxyfuel combustion). Gas washing using BASF's OASE® gas washing process then completely removes CO2 from the flue gas. To ensure that its carbon is not lost but is available again for methanol synthesis, the captured CO2 is fed back into the process. Additional hydrogen is required as a supplement, which should also be produced without CO2 emissions.
