Media
Reversing the aging pocess with epigenetics?
Unlocking the secrets of aging using epigenetics
Everyone wants to live a long life – preferably without the visible signs of aging. Since the dawn of time, humans have pondered the mystery of why we age. Modern science is starting to decipher the biochemical mechanisms behind this process. At the center of this research lies epigenetics – the field of biology that studies how genetic activity is regulated. How do these processes contribute to aging? And, perhaps more importantly: Can we slow them down or even stop them?
Key points at a glance:
-
Researchers are working to decipher the aging process. Mutations in the genome were long considered the main cause of aging, but there are other mechanisms at play.
-
Epigenetics studies explore how chemical tags on DNA influence gene expression.
-
New research is utilizing mRNA technology and other methods to slow down or reverse the aging process.

Fit well into old age
Jeanne Calment was a constant source of astonishment to those around her: The French woman took up fencing at the age of 85, she was still riding a bicycle at the age of 100 and she walked everywhere, attributing her enduring fitness and vitality to all these factors. It truly was enduring: When Calment died in 1997, she was 122 years old, making her officially the oldest person who ever lived.
So, what was her secret? What is it that keeps people fit long into old age and how can we utilize this knowledge? Scientists around the world are grappling with questions such as these. To answer them, it is first necessary to decipher the aging process.
For a long time, it was assumed that aging was mainly caused by mutations in the genes. Over time, these minute changes in the DNA code can disrupt the normal functions of the cells and ultimately cause cell death. The fewer functioning cells we have in our body, the frailer and sicker we become, rather like a car that stops working because of the wear and tear on its components.
But there are animals and people who remain fit and healthy despite these mutations, in many cases appearing much younger than their actual age. This led scientists to conclude that there must be another mechanism at play that influences how we age.
In the 1940s, the British professor Conrad Waddington dedicated himself to this emerging field. Called epigenetics, it focuses on gene properties that come not from the DNA but rather from chemical tags attached to the genes. To illustrate this concept, picture our genes as a collection of books in a library. Some books are marked with small notes – corresponding to the biomolecules – that determine whether a book is being read (gene activated) or not (gene deactivated). These markers are referred to as the epigenetic code. The specific epigenetic code that is established in an individual and whether it changes over a lifetime is defined by the body’s own signaling chemicals and by environmental influences. From air quality and nutrition to stress, exercise and even trauma, these and other factors can trigger biochemical changes and result in corresponding tags on the DNA. With the help of epigenetics, scientists can explain why, for example, only one of two identical twins develops a disease like diabetes; in other words, what activates or deactivates the corresponding genes in only one twin.
Your “real” age doesn’t have to match your age in years.“
What is epigenetics?
A human being has around 25,000 genes, which are not all active all the time. Chemical processes in the body can switch them on or off. Possible triggers can include stress or diet.
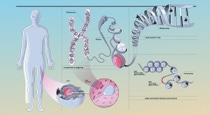
To be or not to be
Each of the individual blueprints for an organism is stored in a specific part of the DNA, the gene. But not all genes are active. Which genes are activated, or which blueprints dictate the production of proteins, enzymes, hormones, etc., changes throughout a person’s life. Genes are deactivated by chemical processes such as DNA methylation: Small molecules that consist of one carbon and three hydrogen atoms attach themselves to certain genes and deactivate them.
A question of genetics
Our genetic material, deoxyribonucleic acid (DNA), is found in the nucleus of each cell in the body. It is distributed over 46 chromosomes. If these were pulled apart and laid end to end, they would stretch out to six feet. The DNA is coiled around proteins called histones, so it can fit into the tiny cell nucleus.
Gene activation through acetylation
Other markers, in turn, can activate genes: Small molecules called acetyl groups are attached to the DNA during acetylation. This releases the genes from their histone wrapping, making the DNA accessible and able to be read.

Epigenetically examined: How old are we really?
In the United States in 2013, working in his lab at the University of California in Los Angeles, Dr. Steve Horvath discovered that many of the markers systematically change their position as people get older, switching certain genes on and switching others off. Based on these changes, he established the first procedure for determining a person’s biological age. Known around the world as “Horvath’s clock,” it uses an algorithm to determine the vitality of an organism, or its “true” age, which does not necessarily have to correspond to its age in years. Horvath’s mathematical model uses certain epigenetic methylation markers (see infographic above) to predict a person’s age: At 50, have we already aged an extra ten years? Or do we have the cells of a 40-year-old? According to the scientist, the blood tests have an accuracy of around 3.6 years.
Since then, other epigenetic clocks have been discovered as well. Some show the age of a specific organ, others are even said to predict a person’s approximate end of life. “Yes, that sounds scary,” Horvath admits. “But this kind of information can be an incentive to make a change in your life.”
It is possible to influence the methylation patterns, giving us a chance to slow down or even turn back our epigenetic clock. Studies show that a diet rich in fruit, vegetables, fish and healthy fats can switch off genes that speed up aging. The biological age of participants who followed the Mediterranean diet was on average 18 months younger than that of those whose diet contained animal-derived proteins and fats, and foods containing sugar. Sufficient sleep and regular exercise also have a positive effect.
New insights from epigenetic research
Professor Vittorio Sebastiano from Stanford University in the U.S. was able to pinpoint other ways in which aging could be slowed down by harnessing mRNA technology to potentially extend our lives. Messenger ribonucleic acid (mRNA) is inside every cell in our bodies. This messenger molecule transports genetic information from the cell nucleus to the parts of the cell where proteins are formed. The researcher injected prematurely aged mice with an mRNA blueprint for a specific repair protein. This enters the cell, where it can mend the epigenetic markers in areas that have been altered over time. In Sebastiano’s experiments, the muscles and eyesight of weakened and blinded animals were restored. The process even offered a way to rejuvenate human skin, muscle and blood cells – by between one and a half to three and a half years.
The aging process was actually reversed.“
Similar results emerged from a study conducted in the U.S. by Dr. Greg Fahy and Robert Brooke, whose biotech company Intervene Immune is a pioneer in biological rejuvenation. The scientists used an experimental anti-aging mix to treat nine men ages 51 to 61 over the course of a year and then analyzed their blood with Horvath’s epigenetic clock. The result: From a biological perspective, the men were on average a year and a half younger after the treatment than when they started, which means that their aging process had not just been slowed, but was actually reversed.
These results were due to a mix of three components: the human growth hormone HGH, the sex hormone precursor DHEA and the diabetes drug Metformin. This combination stimulates the growth of the thymus, the part of the immune system that usually atrophies in older people and is no longer active. In young people, however, the immune organ beneath the breastbone helps to prevent inflammatory processes that accelerate cell aging and diseases.
“We were able to reproduce the rejuvenating effects of the treatment on the immune system in the larger TRIIM-X study and also reverse biological age,” Brooke explains. The reproducibility of the results still needs to be tested in larger studies, but the scientists have taken a major step forward.
This means that living longer – while remaining healthy and fit – is no longer a utopian dream. Epigenetic research is progressing rapidly and delivering new methods to reverse cell aging. Someday, it may well unlock the key to the long life of people like 122-year-old Jeanne Calment, whose own explanation for her longevity was a little less scientific: “God simply forgot about me.”