Developing the batteries that will drive the future

At the Amagasaki Battery Materials Lab in Japan, BASF researchers are developing innovative materials that will improve lithiumion battery performance and increase the driving range of electric vehicles.
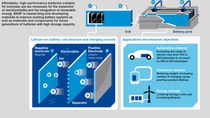
Monday morning 9 a.m., Dr. Masaki Sekine, an organic chemist, sits down at his computer to read the e-mails he has received from colleagues he is collaborating with in Germany and elsewhere in BASF’s international network of R&D battery materials facilities. Sekine works in the newest addition to this network, the Battery Materials Lab in Amagasaki, Japan, inaugurated in early 2014. He is expecting feedback from colleagues on his efforts to create “a totally new molecule that has never existed before.” Sekine is part of the team that synthesizes innovative substances for use as additives in electrolytes, the chemical medium that allows the flow of lithium ions between a battery’s positive electrode, the cathode, and its negative electrode, the anode. The new additives influence the electrochemical reactions in the battery and can therefore affect the battery’s performance.
The kinds of additives Sekine creates depend on which aspect customers want to enhance in their batteries. Many of the lab’s customers are Japanese manufacturers of lithium-ion batteries who are developing new batteries for electric vehicles. They are looking for improvements such as higher power density, greater high temperature stability or an increase in cycle performance, meaning the number of possible discharges and charges. To create a new additive he uses a process of organic synthesis. The synthesis of the additive takes place in a solvent. This solvent must be separated after the successful reaction.
“Purification of the additive is a challenging part of the process,” says Sekine. “In many cases it doesn’t go as planned. And in most cases a single purification round doesn’t provide enough purity to satisfy the strict specifications for a battery additive. Even tiny impurities can lead to uncontrolled reactions and could damage the battery performance significantly, such as increasing the risk that the battery discharges when not in use.” Working in a global network is stimulating and helps teams to find solutions quickly. “I was once stuck with a synthesis,” Sekine recalls. “Theoretically, several synthetic routes were available, but I couldn’t make it work. Then a colleague in Germany contacted me and suggested an alternative catalytic route which was much more efficient.” Such feedback and open collaboration are key. That is why the next step for Sekine is to discuss his results with the lab’s electrochemical testing group.
Testing 1, 2, 3, 4 ...
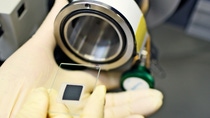
One member of the testing group is Dr. Zhen-Ji Han, who hails from Yanji in China and joined the team in 2013. She helps develop electrolyte formulations and conducts electrochemical testing for batteries using next-generation anode materials. It is her job to test the latest synthesized additives. She takes the new additive to the Cell Assembly room where, donned in coveralls, protective gloves and safety goggles, she mixes it into a prepared base electrolyte and then injects the solution into a test cell. This enables her to examine the cell’s electrochemical properties. Battery performance deteriorates with usage but also over time. Each of these aspects, performance, calendar life and cycle longevity, has to be tested. To test the latter one, the cell is placed in a cycler machine, where it is repeatedly charged and discharged over a period of weeks or months.
Han discusses her findings with the synthesis group. “Because we know more about the battery’s chemical interactions, we can advise the synthesizers which parts of the additives are functioning well or not, and how best to redesign their molecular structures,” she says.
“Our expertise in cathode and electrolyte materials development and our ability to work together as one team differentiate us.”
Dr. Martin Schulz-Dobrick, Head of Amagasaki Battery Materials Lab, Japan
Meeting different needs
A lot of the work carried out at the lab is tailored to individual customers’ requirements. This is where Hiromu Sugiyama enters the stage. The senior researcher in cathode development works directly with customers helping them to achieve performance targets. This involves checking cathode materials’ test data to see that the transition metal content is suitable for the targeted performance tasks, or looking at whether a material’s particle size and shape will enhance or hinder a performance goal. “We always have to consider how the cathode interfaces with the other components in the battery,” explains Sugiyama, who joined the team in 2014. “So testing and analysis is done working closely with the other research teams.”
All under one roof
“The Amagasaki lab is special in a number of ways,” says Dr. Martin Schulz-Dobrick, head of the facility. “It’s BASF’s first facility in Asia Pacific to combine development of cathode materials, electrode and electrolyte materials with application technologies. We not only conduct basic research on these materials starting from scratch, but also support our customers in prototyping batteries.” One example of the application work on a customer’s specific needs is the joint development of an electrolyte recipe. “We are jointly designing and building test batteries, which allow us to test our materials under conditions close to our customers’ needs and therefore speed up the whole devel- opment,” the lab head explains.
And, significantly, the facility brings the different areas of research together under one roof. “This is the big advantage of our lab,” says Schulz-Dobrick, who transferred from BASF’s headquarters in Germany to Japan in 2013. “Our expertise in cathode and electrolyte materials development and our ability to work together as one team differentiate us from other material suppliers, who can generally provide battery makers with either electrolyte or cathode materials. We specialize in both.”




.jpg)



